During the Covid-19 pandemic, a rare, fungal infection called mucormycosis exploded across India. Fungi are not common human pathogens. However, complex factors have been driving up mucor infections, especially in India and China, over the decade. While the global incidence of mucormycosis was around 1.7 cases per million population before 2020, India’s incidence rate was around 140 cases per million population. This was about 80 times higher than the prevalence in developed countries, but the disease burden handled by the country’s health systems made mucor infections a rare disease.
Before the pandemic, people with compromised immunity, either because of disease (like HIV) or chemotherapy, were the most likely to pick up a fungal infection.
Covid-19 accelerated the incidence of mucormycosis. By November 2021, India’s official estimates reported over 50,000 cases of mucormycosis. It became known on the news as the “black fungus” disease because of the black, necrotic tissue it left behind. While Covid-19 was a “pandemic” (a disease or infection that explodes across large regions), in India, it also triggered a mucormycosis “syndemic”—a disease that received a synergistic “boost” from the ongoing pandemic.
Even with new data on excess deaths during Covid-19, there is little collective reckoning and public awareness to prevent future syndemics. Similarly, a true estimate of the prevalence of mucormycosis during Covid-19 has never been published. Nor is there any surveillance or population-level data for mucormycosis from after the pandemic subsided.
Despite these lacunae, the pandemic spotlighted the silent spread of diabetes in India. It underscored the strong association between diabetes and mucormycosis, and showed that our health systems are not ready for a future crisis that trips on these fault-lines. However, this also means that we now understand mucormycosis better and are more effective in treating patients with diabetes who are increasingly developing this infection—a wholly avoidable condition.
Mucor and Infection
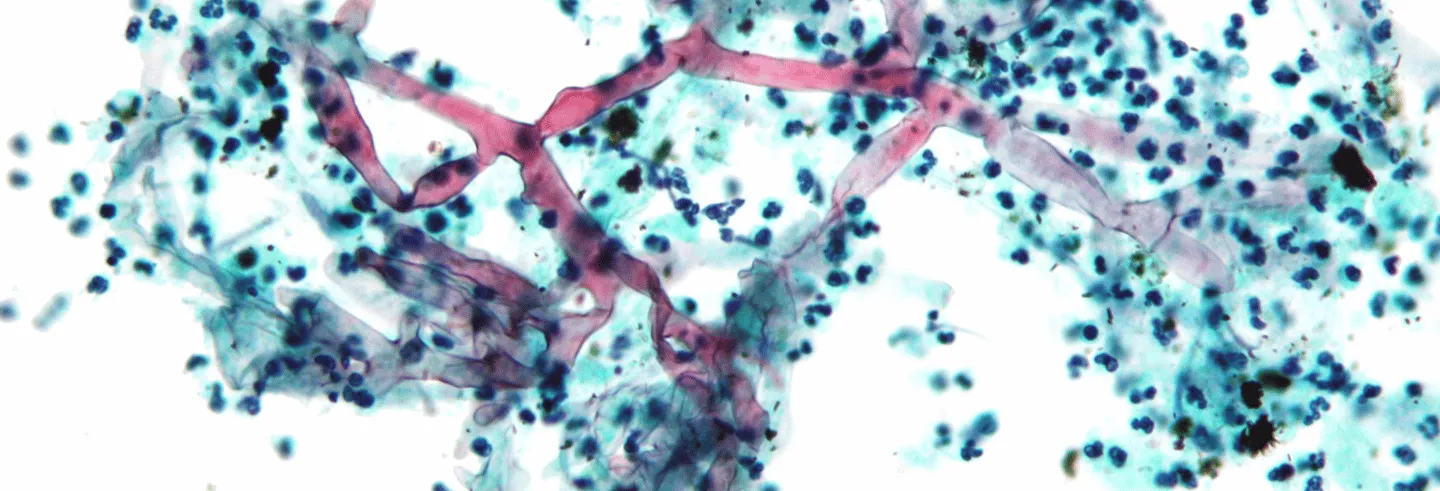
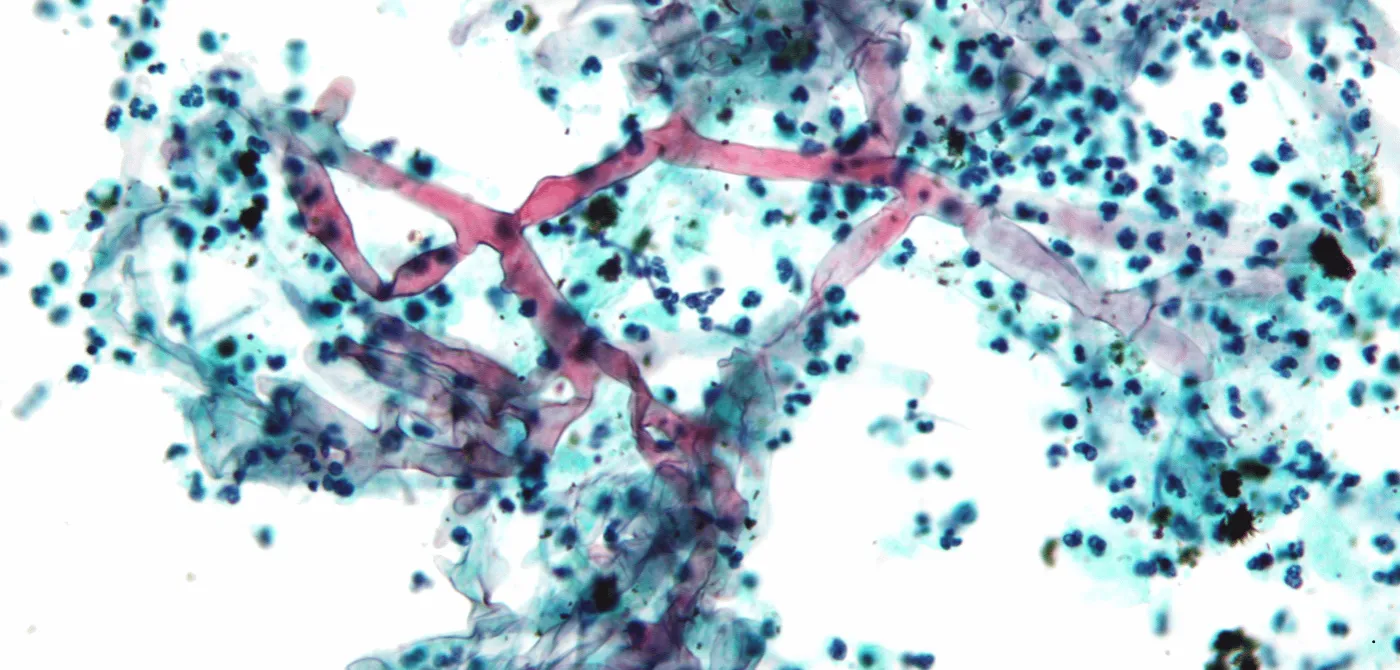
Mucorales are ubiquitous soil moulds that break down organic matter. Many sub-species of fungi come under this family, though some 38 genera are human pathogens. Mucormycosis is a rare and opportunistic fungal infection that is caused by mucorales spore finding a foothold in the human body—commonly in the nasal mucosa (when it is known as Rhino-Orbito-Cerebral Mucormycosis; ROCM), but also lungs, skin, or the gastrointestinal tract. ROCM is very aggressive—spreading to the brain in hours in some cases—and has a 50% fatality rate.
In almost all of us, any large particles that land in our noses are trapped by hair-like structures and transported down via mucus to be cleared away through the stomach. Microscopic fungal spore are attacked by phagocytes, a type of immune cell that engulfs and ingests them. So, mucorales in the air have no effect on most people. Before the pandemic, people with compromised immunity, either because of disease (like HIV) or chemotherapy, were the most likely to pick up a fungal infection. Patients with a severely impaired immune system end up having depleted white blood cells and phagocytes, and are vulnerable to an opportunistic infection by fungi.
Covid-19 infections increased the risk of a runaway immune response and corticosteroids were necessary to reduce this risk in a narrow band of patients with severe or critical Covid-19 infection.
There is one more sub-population who have a compromised immune system—diabetics. Before the pandemic, a majority of patients with mucormycosis were diabetic. Mucormycosis as a consequence of diabetes is an extreme outcome and yet, the epidemiological evidence linking these two outcomes, especially in India, is strong.
Mucorales are very efficient in sequestering free iron, an essential component of enzymes across many life forms. The presence of a rich source of iron enables their growth. When mucor spores infect the nasal mucosa and succeed in overwhelming the body’s immune response, they are able to invade blood vessels coursing through the face—human blood is a great source of iron. From the nasal cavities, the fungi spread through blood-rich, soft tissue to the eyes and the mouth, and finally to the brain.
Another key driver of a mucor infection is glucose, the primary source of energy for all life. People with uncontrolled diabetes mellitus have glucose-rich blood because the glucose is inefficiently cleared away by their bodies. So, blood that is rich in iron and glucose enables the growth and spread of the fungi deep into the facial tissue. This preference for blood makes mucor “angio-invasive”.
And just to make things extra bad, the fungi produce a “virulence factor”, keto-reductase, which enhances these growth factors. Keto-reductase makes the infected environment more acidic too, which is the ideal pH for fungi to flourish. This sets the stage for a fast-acting infection that leaves behind characteristic dead, black-coloured tissue, necrotised by a lack of blood supply.
Pandemic a Risk Multiplier
Many patients, especially those who were hospitalised during Covid-19’s second wave were prescribed—perhaps indiscriminately—methylprednisolone or dexamethasone to lower mortality risk. These are corticosteroids that modulate and tamp down the body’s immune system. Covid-19 infections increased the risk of a runaway immune response, a “cytokine storm”, and corticosteroids were necessary to reduce this risk in a narrow band of patients with severe or critical Covid-19 infection. However, it is likely that these steroids were over-prescribed and that played a role in the explosion of mucormycosis cases. These steroids also result in elevated blood sugar levels, compounding the infection risk.
Some reports noted that a few people who developed de novo diabetes after a Covid-19 infection had not received any steroids. So, a Covid-19 infection itself seems to have been “diabetogenic” in these cases. We now know that there was an undercount of Covid-19 induced mortality in India during the pandemic years. It will be even harder to ascertain the morbidity, including how much of the population has systemic conditions triggered by Covid-19 since 2021.
These factors do not explain the rising cases of mucormycosis from before the pandemic. One of the reasons fungal infections are rare in humans, when compared to bacterial or viral infections, is because fungi prefer cooler environments—around 27 degrees Celsius, while the human body’s mean temperature is around 37 degrees (or about 98.6 degrees Fahrenheit). However, mucorales live in and decompose organic matter into compost in the presence of oxygen, so they operate in a range of temperatures—up to nearly 50 degrees Celsius.
Global warming seems to be driving selection pressure on fungi that are better suited to survive warmer temperatures. This means human beings (and other mammals like cows and pigs, which are key livestock) are at an increasing risk of fungal infections from a set of fungi—Candida, Aspergillus, and Mucorales among others. In 2022, the World Health Organization published its first-ever list of “priority” fungal pathogens “that represent the greatest risk to public health”.
Uncontrolled Diabetes is Danger
The singular risk lurking in all these contexts is diabetes mellitus. (“Diabetes” is from late Greek, and refers to the tendency to “pass” urine often. “Mellitus” is Latin for “sweet”, a nod to the excess glucose in bodily fluids.) The International Diabetes Federation estimates that India has over 90 million people with diabetes, while an estimated 39 million Indians live with undiagnosed diabetes. Various studies indicate that the prevalence of persistent elevated glucose (or uncontrolled diabetes) in India may be around 60-70%.
Though mucormycosis affects far fewer diabetics than other secondary diseases, it stands out for its horrific prognosis and high mortality rate.
Diabetes exacerbates all the conditions that are favourable for a fungal infection. First, elevated blood sugar levels are the number one predisposing factor for mucormycosis. Two, uncontrolled diabetes can trigger ketoacidosis, a serious complication in diabetes, which impairs phagocyte action and increases iron availability in the blood. Then, if any mucorales happen to gain traction, it can trigger mucormycosis.
Needless to say, developing mucormycosis is just one of the many conditions that are triggered when people live long enough with diabetes. Public health experts around the world are trying to tackle the various long-term complications that can arise from poorly controlled diabetes. This includes secondary diseases, like diabetic retinopathy, which permanently damages vision; nephropathy, which damages the kidneys; increased risk of heart disease, and stroke; and more. These conditions result in morbidity and a loss of function in the affected organs. Though mucormycosis affects far fewer diabetics than these, it stands out for its horrific prognosis and high mortality rate.
Surviving Mucormycosis
While we focus on the 50% or more death rate of this infection, those who survive mucormycosis have to battle for years to reclaim their lives. The main anti-fungal drug that is used to treat mucormycosis, amphotericin B, has a long list of side-effects, including kidney toxicity. A key strategy to tackling a raging mucormycosis infection is the surgical removal of infected tissue, to reduce the fungal load in the body, and to clear away the necrotised portions. This may also be necessary to ensure that anti-fungals reach deep inside the face to stop the infection. Inevitably, this leads to large gaps in the porous bone structure of the face where key tissue is taken away.
Treating mucormycosis is a multidisciplinary exercise where ophthalmology; ear, nose and throat; dental; and plastic surgery specialists must work together to first save the patient and then perform reconstruction surgeries.
During the early stages of the pandemic, for example, exenteration—the full removal of the eye ball—was the preferred strategy to save a person who presented with infection in the ocular orbit. Later, research showed that many patients survived with debridement—tissue removal—alone, exenteration did not improve the survival odds. Similar surgeries for other parts of the face—a maxillectomy or a palatal resection—where parts of their nose, palate, ocular orbit, or skull were removed have been refined to ensure the fungal load decreases without too much tissue loss. However, these survivors must now live with a deep disfigurement of their faces.
Treating mucormycosis is a multidisciplinary exercise where ophthalmology; ear, nose and throat; dental; and plastic surgery specialists must work together to first save the patient and then perform reconstruction surgeries. These surgeries are to help patients regain some of the functions lost with the debridement, like chewing or even holding up the eye ball, along with addressing aesthetics. The reconstruction is truly that—metal to augment the skull and to act as a scaffold, plastic and tissue grafts to fill up and give some form and structure, and prosthetics to support a function or address the person’s self-image. In some cases, the facial reconstruction can take many years to complete.
Lessons for the Present
The pandemic bunched together time. It magnified what was on the slow burner—the increasing prevalence of diabetes and our inability to keep it under control in the long term—and showed us some of its terrible consequences with the mucormycosis syndemic. Like all such horrors, this bunching of time also had a silver lining. With so many cases to handle, our treatment strategies became better. Our technologies improved.
Take Magnetic Resonance Imaging (MRI), which has now become the cornerstone of mucormycosis management. MRI is ideal to resolve the soft tissue on the face with great depth and detail and is very sensitive to small changes in the facial tissue due to necrosis or infection. These scans are detailed enough to support high-precision surgery that can reduce disfigurement.
These tools and techniques have not fallen into disuse after the pandemic subsided. This is because of a steady stream of patients—nowhere near the large numbers during Covid-19, but enough to populate dedicated clinic days at hospitals—with diabetes continuing to develop mucormycosis. It is worth stressing again that people with diabetes developing a mucormycosis infection is an extreme and wholly avoidable outcome.
The link between these two conditions is arguably strongest in India. For reasons that are shrouded in what the public health experts call the “social determinants of health” (poverty, caste, gender, and other such factors), the prevalence of diabetes continues to increase in India. Many people develop diabetes without realising it, and by the time they do, their blood sugar levels are often already difficult to control. Our cities are not designed to support walking or physical activity, and high-calorie, sugar-rich foods are easily accessible everywhere. At the same time, healthcare remains both limited and costly. The rise of mucormycosis, a devastating disease, exposes a harsh reality—we are failing to adequately address our nation’s growing burden of diabetes.
Tejah Balantrapu is Associate Director – Science, Health Data, and Storytelling at the L V Prasad Eye Institute and co-leads “SciCity Hyderabad”, an initiative to bring science into the cultural conversations of the city.