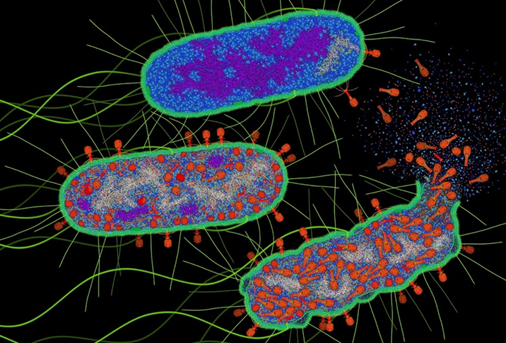
Many of us have seen images of spacecraft like Apollo 11 or Chandrayaan-3 landing on the moon—with their spindly legs and geometrically shaped bodies. Now, imagine something similar happening in the microscopic world—a tiny structure shaped a bit like a lunar lander attaching itself to a bacterium. But this is not a spacecraft—it is a virus. And instead of exploring the moon, it is injecting its genetic material into a bacterial cell.
Because phages can target and destroy harmful bacteria, they are often described as the enemy of our enemy
These special viruses are called bacteriophages, or simply phages. Unlike the viruses that infect humans or animals, phages only infect bacteria. Some types, called lytic phages, go a step further—they kill the bacteria by bursting them open, a process called lysis.
Phages are like tiny molecular machines made of majorly proteins—the same kind of molecules that make up much of our own bodies. Despite their tiny size, they are everywhere. As a matter of fact, phages are the most abundant biological entities on Earth. Just one millilitre of seawater can contain a billion of them. That is more than any other type of organism on the planet.
Because phages can target and destroy harmful bacteria, they are often described as the enemy of our enemy—and that makes them potential allies in the fight against drug-resistant bacterial infections, which is commonly known as antimicrobial resistance or AMR.
India Connection
Phages have an interesting historical connection to India that dates back more than a century. In the early 1890s, a British bacteriologist named Ernest Hanbury Hankin was studying cholera outbreaks along the Ganges River. He noticed something remarkable—the river water seemed to contain something mysterious that could kill Vibrio cholerae, the bacteria responsible for cholera. Whatever it was, it was smaller than bacteria and could pass through fine porcelain filters. Hankin did not know it at the time, but he had stumbled upon bacteriophages.
It was not until about 20 years later, during World War I, that British scientist Frederick Twort and French microbiologist Félix d’Hérelle independently discovered these mysterious bacterial killers. D’Hérelle gave them a name—bacteriophages, meaning bacteria eaters. Almost as soon as they were discovered, researchers began to see their potential as treatments for bacterial infections and started testing them for this purpose.
One of the earliest recorded uses of phage therapy happened in 1919, when d’Hérelle successfully treated a boy suffering from dysentery in Paris using phages. Word spread quickly, and phage therapy was soon being tried in many places—including India, where d’Hérelle reportedly treated 74 cholera patients during an outbreak in Punjab.
But the story took a different turn in the 1930s. The discovery of penicillin by Alexander Fleming and the rise of antibiotics—especially during World War II—shifted the medical world’s focus. Antibiotics were powerful, easy to mass produce, and became the go-to treatment for bacterial infections. As antibiotics flourished through the 20th century, especially until the 1990s, interest in phage therapy declined sharply.
Around this period, before antibiotics were widely available, the Soviet Union continued using phage therapy for a longer time than most countries. However, the approach never really expanded or flourished. Unfortunately, by the time the world began facing the crisis of antibiotic resistance, this weapon in our arsenal had largely been forgotten.
Beyond Antibiotics
Antimicrobial resistance (AMR) happens when microbes—like bacteria—no longer respond to the antibiotics meant to kill them, making infections much harder to treat. It is often called the “silent killer”, because it doesn’t spread in sudden waves like an outbreak, but quietly turns routine infections into serious, sometimes untreatable, ones. People often do not realise it is happening until antibiotics fail. It is one of the biggest health threats we face today, pulling us back toward a time that looks a lot like the pre-antibiotic era.
New phages can be discovered and tested as needed, offering a flexible and renewable tool in the fight against resistant bacteria.
In 2019 alone, AMR was linked to nearly 5 million deaths worldwide. And according to the World Health Organization (WHO), by 2050—just 25 years from now—it could become the leading cause of death globally, with up to 10 million lives lost each year. India is especially at risk. As one of the largest consumers of antibiotics, and with gaps in patient care and public health, the country faces a serious AMR threat.
This growing crisis has renewed interest in alternatives like phage therapy. It is already being explored in many parts of the world as a promising tool against drug-resistant infections. In cases of AMR, phages are not just an alternative—they are sometimes the only option that works. Several recent success stories from around the world have shown that phage therapy can save lives when antibiotics fail.
Phages are often described as living medicines. They work very differently from antibiotics, and, in many ways, these differences make them a better option in certain situations.
Developing a new antibiotic can take eight to 10 years and huge investment—only for bacteria to sometimes develop resistance to it within weeks of use. In contrast, finding and preparing a new phage to target a drug-resistant bacterium can often be done in a matter of weeks or months. With trillions of phages found in nature, scientists often describe them as an almost endless resource. New phages can be discovered and tested as needed, offering a flexible and renewable tool in the fight against resistant bacteria.
Phages are also highly specific. That means a phage of one type of bacteria does not bind and infect other bacteria. Therefore, we can use phages that target only the harmful bacteria, leaving the rest of our beneficial gut bacteria unharmed. Antibiotics, on the other hand, can wipe out both good and bad bacteria, often leading to side effects such as diarrhoea or digestive issues.
And another plus—phages are generally well tolerated by the human body. They are cleared by the immune system without causing allergic reactions in most cases, making them a potentially safer option in some situations.
Another remarkable advantage of phages is their auto-dosing ability. Once they find their bacterial target, they do not just attack—they multiply right at the site of infection. In other words, the treatment strengthens itself as it works. This means that smaller doses are often enough, unlike antibiotics, which usually need to be given repeatedly to stay effective.
Just like with antibiotics, bacteria can develop resistance to phages too. But here is the key difference—phages can also evolve. That means they can adapt in response to changes in the bacteria, making them effective again. It is a kind of microbial arms race—but one that works in our favour.
Scientists are working to uncover exactly how phages recognise and bind to bacteria, what molecules are involved, and how the genomes of both phages and bacteria shape the outcome.
With all these advantages, phage therapy has the potential to become not just an alternative, but a mainstream treatment alongside antibiotics. Still, we are in the early stages of answering many basic questions. Each new discovery brings us closer to making phage therapy a reliable option.
For example, one such question is about their extreme specificity. Phages can sometimes target only a single bacterial strain. While that is a big advantage in avoiding harm to beneficial bacteria, it can also be a drawback. A phage that works for one patient’s infection might not work for another person with a slightly different strain of the same bacterium. This may happen because bacteria carry genetic defence systems, or because small changes in the bacterial cell surface prevent the phage from attaching.
That is why fundamental research is so important. Scientists are working to uncover exactly how phages recognise and bind to bacteria, what molecules are involved, and how the genomes of both phages and bacteria shape the outcome.
To use phages more widely and reliably, scientists also need a better understanding of how they behave in the human body—this includes how they move, how long they can last, and how effectively they reach the infection site. It is also important to fully understand their safety and how well they work.
Phage Therapy Case
In recent years, several successful 'compassionate use' cases of phage therapy have drawn attention—not just from researchers, but also from the wider medical community. These are cases where phages were used as a last resort when antibiotics failed.
So how does phage therapy actually work when a patient runs out of antibiotic options? How does a phage go from the lab bench to the hospital bedside?
One example case can be drawn from Graham Hatfull, a scientist and phage expert at the University of Pittsburgh. In 2018, his team helped treat patients with cystic fibrosis who were battling a drug-resistant Mycobacterium abscessus infection—a notoriously difficult bacterium to eliminate. When doctors ran out of options, they reached out to Hatfull, asking if he could find phages that might work against the infection.
Fortunately, he had just the right resource—a massive library of phages stored in the freezers of his lab. Over the years, he and his team had collected thousands of phages from places like backyard soil, pond water, and even seawater. What is remarkable is that much of this work was done in collaboration with high school and college students, as part of an educational programme that gives young people hands-on research experience. What started as a teaching initiative had turned into a real scientific resource—one that is now helping save lives.
Only the most effective phages—those that wipe out most of the bacteria with minimal resistance—are chosen for therapy.
Once, the patient’s bacterial sample is sent to the lab, the bacteria are grown on Petri dishes and in liquid cultures to study their behaviour and identify them clearly. Then, a lawn of bacteria is spread on a dish, and different phages are placed on top to see which ones can infect and kill them. If a phage works, it creates a clear spot—called a plaque—showing it has successfully infected the bacteria.
But that is just the first step. Next, scientists test how well the phage kills the bacteria and whether any resistant bacteria survive. Only the most effective phages—those that wipe out most of the bacteria with minimal resistance—are chosen for therapy.
Once a promising phage is found, it is grown in large quantities, purified, and carefully measured. The final preparation can then be given to the patient, either through an injection or applied directly to the infected area. Luckily, phages can be multiplied easily, making them a practical option when time is critical.
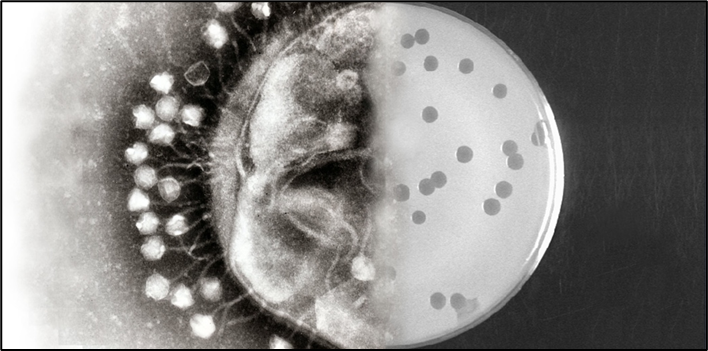
Around the world, phage therapy is currently being explored in two main forms—phage cocktails and engineered phages. Phage cocktails are mixtures of different types of phages that can target the same or similar types of bacteria. Think of it like a team of specialists, each with slightly different strengths, working together to make sure the harmful bacteria are eliminated. This approach is useful because bacteria are good at evolving resistance—so using multiple phages at once makes it harder for them to escape treatment.
On the other hand, engineered phages are custom designed in the lab. Scientists tweak the genetic material of a phage to improve its ability to kill bacteria or to overcome resistance. In some cases, phages can even be programmed to deliver special enzymes that help break down bacterial defences. This approach is still in its early stages but holds a lot of promise for treating infections where natural phages are not effective enough.
Using these approaches, phage therapy has been used to treat a variety of hard-to-cure infections, including lung infections, urinary tract infections, heart infections, and prostate infections, among others. Most often, it is aimed at bacteria that are commonly picked up in hospitals—dangerous strains that no longer respond to many antibiotics. In fact, many of these bacteria are listed on the WHO’s 2024 priority pathogens list, which highlights the most urgent drug-resistant threats to human health.
Common Framework Needed
Unfortunately, most reported cases of phage therapy still happen under compassionate use, in special collaborations between scientists and physicians. In parts of Europe, though, phage therapy has a much longer history. The Eliava Institute in Georgia, for instance, has been working with phages for more than 100 years and is recognised today as a global hub for phage research and treatment. Many hospitals and private organisations now work with the Eliava Institute to expand access, but the overall scale and reach remain limited.
Most people have never even heard of phage therapy, let alone considered it as an option when antibiotics fail.
At present, some guidelines are available in the United States and Europe that allow doctors to apply phage therapy in certain situations. However, a common international framework has yet to be established. To take phage therapy beyond just compassionate cases in treating antimicrobial resistance, stronger regulations and broader access will be needed.
For example, in Belgium, phage researcher Jean-Paul Pirnay and colleagues have set up a system where phage preparations can be made by hospital pharmacists and prescribed directly to patients. This approach avoids some of the heavier regulatory hurdles while still ensuring safety. On a larger scale, an international consortium called Phagistry has been created to systematically record phage therapy cases and collect important data on diagnoses, phages used, treatments, and patient outcomes. Efforts like these are helping to lay the foundation for future global guidelines for phage therapy.
Like any medicine, phage therapy must meet basic requirements of safety and effectiveness before it can become a routine treatment for drug-resistant infections. Early reports suggest that phages are generally safe, with very few side effects, but much more evidence is needed. Standard studies—such as pre-clinical and toxicity tests, good manufacturing practice (GMP) certification, and large-scale randomised clinical trials—are essential.
For readers who want to explore more inspiring stories of phage therapy, a good place to start is The Perfect Predator. Written by infectious disease epidemiologist Steffanie Strathdee, the book recounts her personal journey of how phage therapy saved her husband’s life when a deadly, antibiotic-resistant infection left doctors with no options. The story not only captivated readers but also helped reignite serious interest in phage therapy among scientists, clinicians, and policymakers in the West. Another excellent book is The Living Medicine, written by Lina Zeldovich, a journalist. It traces the fascinating history of phage therapy against a backdrop of geopolitics and scientific rivalries, with the twist and turns of a thriller. It also offers insight into both the promise of phages and the real challenges researchers and doctors face in bringing this therapy from the lab bench to the patient’s bedside.
In recent years, such studies have been increasing, which is encouraging. The hope is that this research will help establish clear, universal guidelines and regulations, paving the way for phage therapy to move from experimental and compassionate use into mainstream medicine.
Another big challenge is awareness—or rather, the lack of it. Most people have never even heard of phage therapy, let alone considered it as an option when antibiotics fail. This gap makes it all the more urgent to spread the word and build the systems needed to make such life-saving treatments available to those who need them.
Advocacy is key. It can begin with simple efforts like social media campaigns and outreach in schools and extend to medical education through seminars and conferences for doctors. Raising awareness at every level—from the general public to healthcare professionals—will be essential.
Potential in India
In India, bacteriophage research is still at a very early stage and needs to grow quickly to keep pace with global developments. Only a handful of research labs are working on different aspects—such as isolating and studying new phages, exploring their use in clinical trials, developing treatment strategies for drug-resistant infections, and building phage banks.
On the research side, phage science is getting a big boost from recent advances in cutting-edge technology. For example, rapid genome sequencing now allows scientists to quickly identify and catalogue new phages for building up phage banks. Tools like cryo-electron microscopy and tomography let researchers see these tiny virus particles in incredible detail—down to the atomic level—and understand exactly how they infect bacteria.
India is considered one of the richest natural sources of phages, thanks to its diverse environments. This presents a unique opportunity. With the right support…the country could play a key role in advancing phage therapy.
All this information is feeding into machine learning and artificial intelligence systems, which could one day help us design and create tailor-made phages to target specific bacterial infections. That is the vision many leading phage scientists around the world are working towards. For India to stay in the race, it is important to adopt and invest in these advanced tools and technologies. They are key to unlocking the full potential of phage therapy.
Interestingly, India is considered one of the richest natural sources of phages, thanks to its diverse environments. This presents a unique opportunity. With the right support—such as dedicated funding from major science agencies, improved infrastructure, and stronger collaborations between academia and industry—the country could play a key role in advancing phage therapy.
Finally, there is also strong potential for the growing start-up ecosystem to take interest. With promising applications in healthcare and biotechnology, phage therapy could become a vibrant area of innovation in India’s pharma and biotech sectors.
Sudipta Mondal is a postdoctoral research associate at Scripps Research in San Diego, United States of America, where he studies how phages interact with bacteria. He obtained his PhD from the CSIR–Centre for Cellular and Molecular Biology, Hyderabad.









