Change is the only constant – this is especially true when it comes to antibiotics. We have gone from benefiting immensely from their discovery, to misusing them, and now to realising the importance of preserving their effectiveness for our own well-being.
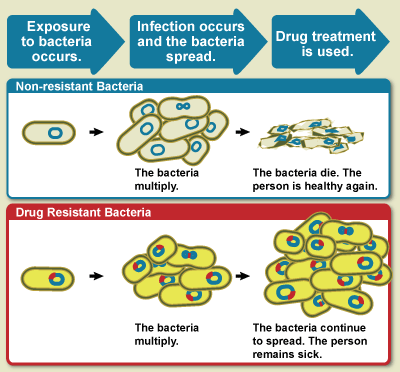
Antibiotics are a class of widely used antimicrobial drugs. They kill or stop the growth of bacteria. These drugs have been crucial in fighting bacterial infections such as tuberculosis, urinary tract infections, and sexually transmitted diseases. However, these drugs are becoming less effective because bacteria are evolving. This reduced effectiveness, where antimicrobial drugs no longer work against microbes, is known as antimicrobial resistance (AMR). This has now taken the form of a public health crisis in which millions of people are dying due to ineffective treatments. And, unfortunately, overuse and misuse of antibiotics fuels this problem.
Miracle drug
It was in 1928 that the first antibiotic, penicillin, was discovered. In the two decades that followed, it was mass produced industrially and made available for public use as a medicine. This period coincided with World War II, during which antibiotics saved countless lives, both of soldiers and civilians affected by the conflict.
Subsequently, many other antibiotics were discovered and produced. Their use significantly reduced deaths due to infectious bacterial diseases such as pneumonia, diphtheria, plague, and tuberculosis. The global average life expectancy was 30-32 years in the early 1900s. It has now more than doubled, and antibiotics have played a large role in this increase.
Farmers use antibiotics to prevent the spread of bacterial infections, a problem that becomes worse when animals are kept in crowded conditions. They also use antibiotics as growth promoters.
People assume incorrectly but confidently that antibiotics can cure all diseases. They buy them over the counter from pharmacies and share leftover pills or capsules with friends and family for self-medication. When they see their doctors, they often ask for antibiotics. They believe antibiotics will heal them sooner. Doctors and pharmacists comply for fear of competition in their businesses. However, antibiotics do not work against viral, fungal, or parasitic diseases. Most of the time, especially with common everyday illnesses, it is very difficult for someone without medical training or access to reliable diagnostic tools to determine the cause of an infection.
Not just in human healthcare, antibiotics are used in plant and livestock agriculture. They are sprayed with pesticides on plant farms and added to animal feed. Farmers use antibiotics to prevent the spread of bacterial infections, a problem that becomes worse when animals are kept in crowded conditions. They also use antibiotics as growth promoters. It is not fully clear how antibiotics promote weight gain. Some scientists suggest that by reducing the gut microbial population, the animals do not lose nutrients to microbes and gain weight faster.
Antibiotics in environment
The misuse of antibiotics has consequences that go beyond the individual. It is not just about personal expenses or side effects like poor gut health. Overuse of antibiotics results in their high prevalence in the environment, especially in soil and water. This, in turn, creates conditions that favour the growth of bacteria that are more resistant to antibiotics.
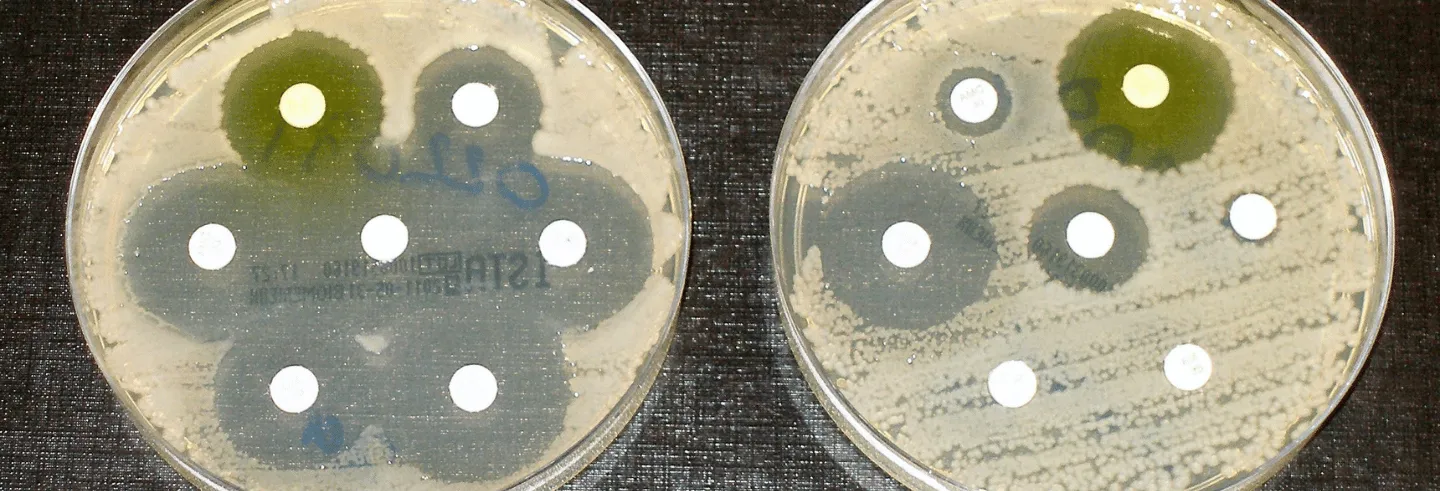
Not all bacteria are equally sensitive to antibiotics, thanks to variations in their DNA. These variations could arise randomly when DNA duplicates in a dividing bacterial cell. They help some bacteria metabolise antibiotics and discard them from their system. Or some variations prevent antibiotics from even entering the bacterial cell. Bacteria with such powers are naturally better equipped to survive in antibiotic-rich environments. They are called antibiotic-resistant bacteria.
Bacteria can share their resistance powers very quickly with other bacteria by transferring their antimicrobial resistance genes. They can do so not only with their next generation of cells, but also with their neighbours through a process called conjugation. Here physical contact between two cells allows the AMR bacteria to take over the available space in an antibiotic-rich environment cleared of all susceptible bacteria.
Despite these staggering numbers, there is hardly any public awareness of this crisis because death reports do not mention AMR as a cause of disease or death.
When one gets infected by AMR bacteria, existing antibiotic treatments fail to work. The treatments become prolonged and more expensive with newer antibiotic cocktails. This affects people’s lives and livelihoods. Similarly, there are anti-virals not working on viruses, anti-fungals ineffective against fungal infections, and anti-parasitics powerless against parasitic infections. But in terms of the damage caused, bacterial AMR tops the charts.
The World Health Organization estimated 4.95 million deaths globally due to bacterial AMR in 2019. The number of deaths a year is expected to double in the next two decades if the present trend continues. Those with low immunity such as newborns, the elderly and the immune-compromised; women who frequently contract urinary tract infections; and those living in areas with a high tuberculosis burden or anyone who may need to spend longer periods in hospitals, are at a greater risk of AMR infections.
Despite these staggering numbers, there is hardly any public awareness of this crisis because death reports do not mention AMR as a cause of disease or death. Instead, the reason cited would be a more immediate cause such as organ failure. Also, unlike Covid-19 and other infectious diseases, AMR is not the result of infection by one specific microbe. So, the problem seems too big to tackle.
The problem also seems gigantic because of the number of stakeholders who have a role to play in dealing with it. They include the manufacturers, prescribers, sellers, and users of antimicrobials and the agencies that oversee the compliance of all involved in the supply chain.
Fighting antimicrobial resistance
When the existing antibiotics become ineffective against bacteria, we do not have the easy option of picking a new drug. That is because designing and testing new antibiotics is a hard and slow process. It also does not help that developing new antibiotics is not a priority amongst pharmaceutical companies because it is often not profitable for them.
This puts countries like India where infection loads are high in danger. In addition to treating infections, antibiotics are widely used in childbirth, dental procedures, surgeries, chemotherapy, and other conditions where immunity is compromised. So, stopping the use of these drugs altogether is not a wise solution. But using them responsibly to reduce their concentrations in the environment is. The less frequently bacteria are exposed to antibiotics, the less advantage antibiotic-resistant bacteria have over other bacteria. As long as the bacteria around us remain genetically diverse, we remain safe.
Doctors must also adopt more precise diagnostic tools and collaborate with the hospital’s microbiology department to know which pathogen is prevalent in their localities before deciding which antibiotic to use.
Various stakeholders have to come together to ensure that antibiotics are used sensibly.
Patients should be aware that antibiotics are classified as Schedule H drugs, marked by a red line on their packaging. This means they should only be purchased with a valid prescription from a licenced doctor. Consumers should finish the entire course of the drug even though they might feel better after the first few doses. This ensures that the bacteria exposed to the drug are completely eradicated from their immune system.
Medical doctors, including ayurveda, yoga and naturopathy, unani, siddha, and homeopathy (AYUSH) doctors and registered medical practitioners, have to be responsible in their prescriptions of antibiotics. They should not feel pressured by patients demanding antibiotics and prescribe them only when they are convinced that the infection is bacterial in nature. They should explain how antibiotics work and the importance of finishing a course. Doctors must also adopt more precise diagnostic tools and collaborate with the hospital’s microbiology department to know which pathogen is prevalent in their localities before deciding which antibiotic to use.
For those with limited access to doctors, pharmacists often decide what medicines they take. These pharmacists have to be fully aware of the responsibility of selling antibiotics. Antibiotics are grouped into three categories—Access, Watch and Reserve—reflecting increasing restrictions on their sale. Pharmacists are required to keep meticulous sales records to enable regulatory audits and ensure these medicines are used responsibly.
A study compared bacterial AMR in areas that restricted over-the-counter sale of antibiotics in São Paulo, Brazil. The researchers found a correlation between drops in the sale of specific antibiotics and reduced bacterial AMR against them. Similarly, they also found a rise in bacterial AMR against the antibiotics that had higher sales.
Farmers use the pesticide/feed mix they get from their suppliers and there have been no regulations on how much antibiotics are to be added to them. Experts believe that antibiotics are used at an excess of almost 75 times in the poultry industry globally. These antibiotics not only reach the consumers of these agricultural products but also mix in water and soil, affecting the overall microbial population. However, in a positive move last year, many governments across the world committed to reducing the use of antibiotics in agriculture by 2030 at the United Nations General Assembly. The Food Safety and Standards Authority of India (FSSAI) has banned the use of antibiotics in any stage of production of milk and milk products, meat and meat products, poultry and eggs, aquaculture and its products. This was to come into practice on 1 April 2025.
Similarly, pharmacies, and ideally the public too, should be able to send their unused medicines for safe disposal to similar facilities.
The other important way of reducing antibiotics in the environment is through safe disposal procedures. This includes mandating antibiotic manufacturing industries and hospitals to incinerate their waste so that antibiotics in them are destroyed. Similarly, pharmacies, and ideally the public too, should be able to send their unused medicines for safe disposal to similar facilities. New initiatives are under way in some places will help citizens to safely dispose of their unused and expired medicines.
Governments have to keep an eye on the rising resistance in bacteria against all existing antibiotics. Colistin, a crucial last-resort antibiotic, has been banned in farm animals in various countries, including India. This has reduced the incidence of resistance against colistin in farm animals. This finding is supported by independent studies from China, Japan, and Portugal. Though this gives us hope that AMR may be reversible, newly approved antibiotics, such as nafithromycin, should be used judiciously right from the start. Since 2017, only 13 new antibiotics have obtained marketing authorisation. Of these, only two represent a new class of antibiotics. This means that only these two might not have existing AMR bacteria against them at the moment.
Amid all this, it is important to remember that the most potent way of fighting AMR is by avoiding infections. Hygiene, good nutrition, and vaccines are well-established ways of preventing infectious diseases and should be in the top of any country’s healthcare goals. Achieving these will restrict using antibiotics only to occasions where there are no other viable options.
Somdatta Karak heads science communication at the Centre for Cellular and Molecular Biology, Hyderabad. She co-leads Superheroes against Superbugs, an educational and public engagement initiative on antimicrobial resistance in India.